Synthesis of isocyanate-free polyurethane concocting multiple cyclic carbonates catalysed by a new microporous zinc phosphonate via CO2 fixation†
Abstract
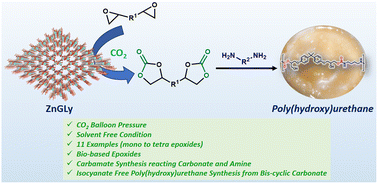
Chemical fixation of carbon dioxide (CO2) on reactive organics is very challenging from the perspective of sustainable synthesis of fine chemicals and mitigation of this abundant greenhouse gas. Herein, we report the synthesis of a novel microporous zinc phosphonate (ZnGLy) using N,N-bis(phosphonomethyl)glycine as a bridging phosphonate precursor under hydrothermal reaction conditions. The high surface area with regular microporous channels, along with the presence of both Lewis acidic and basic sites in ZnGLy, has motivated us to explore its catalytic potential for the chemical fixation of CO2 over a series of reactive organic molecules. ZnGLy exhibited excellent catalytic performance for the synthesis of mono- to tetra-cyclic carbonates, including biologically active epoxides, at moderate CO2 pressure. Utilizing CO2 as a C1 source, this material also displayed excellent catalytic activity towards the synthesis of value added hydroxy carbamates. The bis-cyclic carbonate synthesized herein was further used for the synthesis of isocyanate-free poly(hydroxy)urethane. Furthermore, ZnGLy exhibited outstanding recyclability for several catalytic cycles with the retention of its framework, suggesting its huge potential as a heterogeneous catalyst for large scale CO2 fixation reactions.
- This article is part of the themed collection: #RSCPoster Conference


 Please wait while we load your content...
Please wait while we load your content...