Epoxidation of olefins enabled by an electro-organic system†
Abstract
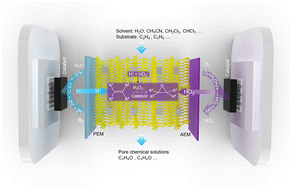
Renewable electricity-driven organic electrosynthesis processes, with enhanced energy conversion efficiencies and reduced carbon footprints, for high-value organic chemicals production are enticing chemists to complement or even supplant traditional industrial synthesis routes. Despite being regularly lauded, electrosynthesis remains a very under-used procedure in organic chemical laboratories and industry. Olefin oxidation chemistry plays an essential role in manipulating organics and the resulting epoxides are widely used as feedstocks and intermediates in industry. Here, we describe an electro-organic system with a phase-separated membrane-electrode assembly strategy, coupling in situ H2O2 electrogeneration and olefin epoxidation, that delivers pure solvent-only epoxide solutions with energy conversion efficiencies >90%, consuming only H2O and O2 under ambient conditions. Specifically, the electrocatalytically generated H+ and HO2−, from H2O oxidation and O2 reduction, are recombined to form H2O2 using an ionic conductor, acting as the oxidizing agent for olefin epoxidation. Using engineered efficient catalysts, we demonstrate broad substrate scope and extendable reactions for downstream organic synthesis involving H2O2.
- This article is part of the themed collection: 2022 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...