An in vitro cascade with four enzymes for the production of d-3,4-dihydroxybutyric acid from d-xylose†
Abstract
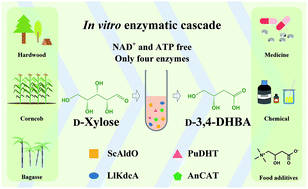
D-3,4-Dihydroxybutyric acid (D-3,4-DHBA) is an important platform chemical with diverse applications in the chemical and pharmaceutical industry. The reported metabolic pathway for D-3,4-DHBA production from D-xylose involves two NAD-dependent dehydrogenase-catalyzed reactions and thus is redox-imbalanced. In this study, the mutant alditol oxidase from Streptomyces coelicolor A3 (ScAldO) was identified to catalyze the oxidation of D-xylose and D-3,4-dihydroxybutanal, the two NAD-dependent dehydrogenase-catalyzed reactions required for D-3,4-DHBA production. Then, an in vitro enzymatic cascade composed of ScAldO, dihydroxy acid dehydratase from Paralcaligenes ureilyticus, α-ketoacid decarboxylase from Lactococcus lactis and catalase from Aspergillus niger was constructed for the production of D-3,4-DHBA from D-xylose. Under the optimal conditions, D-3,4-DHBA at a concentration of 4.55 g L−1 was produced from D-xylose with a molar yield of 83% and a productivity of 0.217 g L−1 h−1. The in vitro enzymatic cascade only requires four enzymes and excludes expensive cofactor addition and thus may be a potential choice for industrial biomanufacturing of D-3,4-DHBA.


 Please wait while we load your content...
Please wait while we load your content...