Sustainable multicomponent indole synthesis with broad scope†
Abstract
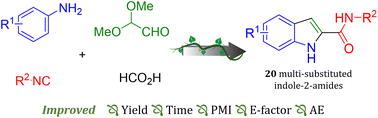
The most preferred heterocyclic indole core was de novo assembled by an innovative 2-step reaction from inexpensive and broadly available anilines, glyoxal dimethyl acetal, formic acid and isocyanides involving an Ugi multicomponent reaction followed by an acid induced cyclization. As opposed to many other indoles syntheses, our method delivers under mild and benign conditions using ethanol as solvent and no metal catalyst. The scope of the reactions was scouted and 20 derivatives are described.
- This article is part of the themed collection: 2022 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...