Substrate promiscuities of a bacterial galactokinase and a glucose-1-phosphate uridyltransferase enable xylose salvaging†
Abstract
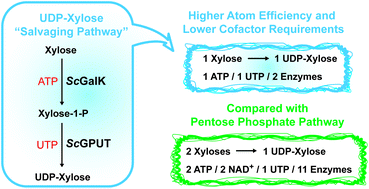
Galactokinases (GalKs) are structurally conserved proteins that exist in all domains of life. These enzymes catalyse the transfer of a phosphate group from adenosine triphosphate (ATP) to the anomeric hydroxyl group of galactose and show only negligible substrate promiscuities toward other sugars such as glucose, mannose, or xylose. Here we describe a peculiar GalK orthologue from the bacterium Solitalea canadensis (ScGalK) with relaxed acceptor sugar requirements, which also converts xylose to xylose-1-phosphate in the presence of ATP. Investigating the surrounding genomic DNA region of the ScGalK gene revealed a putative Glucose-1-phosphate Uridyltransferase (ScGPUT) candidate in close proximity, and the recombinant gene product of ScGPUT was able to convert xylose-1-phosphate into uridine diphosphate xylose (UDP-xylose) in the presence of uridine triphosphate (UTP). Given that UDP-xylose is an essential building block for the generation of xylose-containing glycoconjugates in bacteria, converting xylose using these two enzymes into UDP-xylose significantly reduces the cofactor requirements compared to the known standard xylose regeneration via the pentose phosphate pathway.


 Please wait while we load your content...
Please wait while we load your content...