Accelerated synthesis of dendrimers by thermal azide–alkyne cycloaddition with internal alkynes†
Abstract
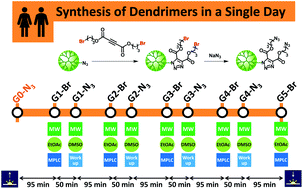
An accelerated synthesis of dendrimers is presented by combining the thermal azide–alkyne cycloaddition (AAC) and azide substitution reactions. The strategy is among the most user-friendly, flexible, modular, and accelerated strategies for the synthesis of dendrimers. Despite AAC being an archetypal click reaction that proceeds with complete atom-economy and is amenable to a high chemical diversity, high temperatures and prolonged reaction times have limited its application in the synthesis of dendrimers. By combining the optimized AAC/azide substitution conditions, simplified workups/purifications, and a strict alignment to green chemistry principles, not only a G5 dendrimer with 96 terminal groups has been synthesized and characterized in less than 12 h, but also the newly formed triazol has been successfully used to promote the easy exploration of the dendritic chemical space, the peripheral decoration of dendrimers, and the straightforward adjustment of dendritic properties.
- This article is part of the themed collection: Bioorthogonal and click chemistry: Celebrating the 2022 Nobel Prize in Chemistry


 Please wait while we load your content...
Please wait while we load your content...