Boosting CO2 electroreduction to C2+ products on fluorine-doped copper†
Abstract
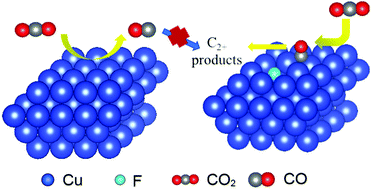
Electrochemical reduction of CO2 by renewable electricity offers a promising way to settle excessive greenhouse gases and achieve carbon neutrality under a mild environment. Cu-based catalysts have a unique catalytic activity towards multicarbon (C2+) products in the CO2 electroreduction reaction and are widely studied to promote the corresponding selectivity and activity. Here we report a F-doped Cu (F–Cu) catalyst to improve C2+ products in CO2 electroreduction. A high C2+ product (mainly ethylene and ethanol) faradaic efficiency of 70.4% was achieved on the F–Cu catalyst with a high current density (above 400 mA cm−2) in the flow-cell system. The in situ surface enhanced Raman spectroscopy results and electrochemical performance analysis demonstrated that CO2 could be reduced into CO at low potentials and the adsorption of CO could be strengthened on F–Cu, which is beneficial to the subsequent C–C coupling step and formation of the C2+ products.
- This article is part of the themed collection: 2022 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...