{2-Phases 2-reactions 1-catalyst} concept for the sustainable performance of coupled reactions†
Abstract
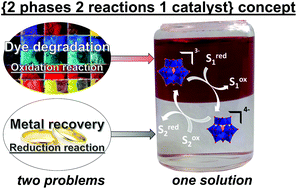
In recent years, photoredox chemistry has been intensively investigated due to its sustainability and low energy-cost. Here, we propose a physicochemical concept – called {2-phases 2-reactions 1-catalyst} – to perform two chemical reactions simultaneously with only one catalyst using a liquid–liquid biphasic system. The well-known and commercially available α-Keggin polyoxometalate (POM), PW12O403−, was used as a photocatalyst for oxidizing a hydrophobic compound in an organic phase. The reduced POM, PW12O404−, being more hydrophilic, diffuses then in the aqueous phase where it reduces a hydrophilic compound. After this step, the re-oxidized POM migrates back to the organic phase closing the catalytic cycle. We applied this concept to several basic reactions: oxidations of organic compounds (e.g. alcohol to aldehyde), degradation of organic dyes, metal recovery by reduction of metal ions, and in situ metal–organic complexation reactions. This concept is proposed as a powerful, simple and sustainable tool to couple diverse photo-oxidation/reduction reactions. As an example, oxidative dye degradation/metal (silver) recovery were performed using this general methodology. The main advantage of the {2-phases 2-reactions 1-catalyst} concept is the economy of one reactor and one catalyst, including its self-regeneration by another useful reaction.


 Please wait while we load your content...
Please wait while we load your content...