Continuous-flow stereoselective reduction of prochiral ketones in a whole cell bioreactor with natural deep eutectic solvents†
Abstract
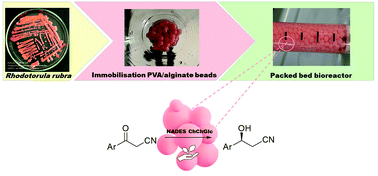
Immobilized whole cells of Rhodotorula rubra MIM147 were used in a packed bed flow reactor for the enantioselective reduction of β-ketonitriles and for the efficient production of a key building block for the synthesis of the antidepressant drug duloxetine. A choline chloride–glucose natural deep eutectic solvent (NADES) was employed with a dual function, as a co-solvent and as a source of glucose, fundamental for cofactor regeneration. To develop a fully automated protocol, an in-line purification procedure was also developed. Firstly, an in-line liquid–liquid extraction of the desired β-hydroxynitriles was performed with a flow stream of ethyl acetate, then the unreacted ketone was trapped by a polymer-supported benzylamine. The optimized protocol allowed the obtainment of (S)-β-hydroxynitriles in 80 minutes of residence time with >95% conversion and excellent e.e. (96–99%).


 Please wait while we load your content...
Please wait while we load your content...