CO2 treatment enables non-hazardous, reliable, and efficacious recovery of spent Li(Ni0.5Co0.2Mn0.3)O2 cathodes†
Abstract
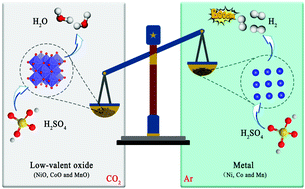
The rapid growth in the demand of lithium-ion batteries (LIBs) will inevitably lead to a shortage of related metal resources. Recycling valuable metals from spent LIBs has become imperative. Here, we propose a non-hazardous, reliable, and efficacious process for in situ recovery of valuable components from spent LiNi0.5Co0.2Mn0.3O2 cathodes. First, the cathode sheets pre-acquired by disassembly were thermally treated under a CO2 atmosphere, and intact Al foils with obvious metallic luster were recovered. The lithium component (Li2CO3) was preferentially dissolved in water, and the recovery efficiency reached 92.84%. Meanwhile, the residues containing Ni, Co, and Mn components were easily dissolved in sulfuric acid solution without any additional heat treatment, and no explosive gases were generated. Then, by comparing the thermal reduction characteristics of cathode materials under different atmospheres (air, Ar, and H2/Ar), the mechanism of pyrolysis of cathode materials under a CO2 atmosphere and the role of CO2 in the thermal reduction process were systematically studied. This work not only provides a novel method for the recycling of spent cathode materials but also guarantees the safety of related personnel and properties.


 Please wait while we load your content...
Please wait while we load your content...