Screening and bioavailability evaluation of anti-oxidative selenium-containing peptides from soybeans based on specific structures
Abstract
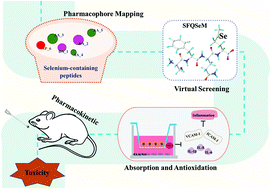
Our previous study has evaluated the antioxidant capacity and identified the sequences of soybean selenium-containing peptides. Herein, pharmacophore screening, gastrointestinal simulation and in vivo pharmacokinetics were performed to predict the potentials of selenium-containing peptides in terms of antioxidant activity, safety and bioavailability. A pharmacophore model with 6 structure features was constructed for virtual screening to determine the potential activities of 85 selenium sequences from soybean peptides. Strong reversing effects (p < 0.05) of the targeted sequences were observed in tumor necrosis factor-α (TNF-α)-induced inflammatory cytokines and adhesion factors burst in EA·hy926/Caco-2 co-culture cell models. Ser-Phe-Gln-SeMet (SFQSeM), a promising peptide selected from both virtual screening and cell models, was proved to be stable in the gastrointestinal tract and could be transported across the Caco-2 monolayer via the paracellular pathway. Additionally, SFQSeM showed a long residence time (89.42 ± 1.34 min) and half-life (81.60 ± 11.88 min) after consumption, and it induced lower liver alanine/aspartate transaminase (ALT/AST) and serum nitric oxide (NO) levels compared to Na2SeO3 and SeMet (p < 0.05). The potency of SFQSeM against oxidative stress as well as its oral bioavailability and low risk highlight its potential utility as an effective Se nutritional supplement.


 Please wait while we load your content...
Please wait while we load your content...