A randomised, double-blind, placebo-controlled trial of Bifidobacterium bifidum CCFM16 for manipulation of the gut microbiota and relief from chronic constipation†
Abstract
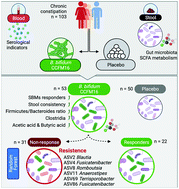
A variety of opinions exist on the potential of probiotics to provide relief from chronic constipation with much focus being placed on their mechanism of action and causes of heterogeneity in the results of different studies. We aimed to determine the efficacy and safety of ingesting Bifidobacterium bifidum (B. bifidum) CCFM16 for 28 days to relieve constipation and to understand the mechanism of action. Using Rome IV criterion, 53 and 50 participants diagnosed with chronic constipation were included in the probiotic group and placebo group, respectively. Spontaneous bowel movements (SBMs) per week, stool consistency (Bristol Stool Form Scale [BSFS]), the proportion of SBM responders, patient assessment of constipation symptoms (PAC-SYM), and quality of life (PAC-QoL) were evaluated. The gut microbiota, short-chain fatty acids (SCFAs) and other indicators were also assessed. B. bifidum CCFM16 treatment improved the stool consistency and increased the proportion of SBM responders, but the differences in PAC-SYM and PAC-QoL were statistically insignificant between the groups. Analysis of the SCFAs and microbiome revealed that CCFM16 significantly increased the acetic acid and butyric acid concentrations and enhanced the Firmicutes/Bacteroidetes ratio. Levels of Clostridia were particularly increased and were associated with the increase in butyric acid. In addition, we found the other side of Clostridia; several taxa in the order Clostridiales were observed to prevent CCFM16 from proper functioning in the pre-treatment microbiome. In conclusion, CCFM16 can potentially and efficaciously relieve chronic constipation in Chinese adults by regulating the gut microbiota and SCFA metabolism. The two sides of Clostridia illustrate its importance in microbial therapy for constipation.
- This article is part of the themed collection: Food & Function HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...