Dynamics over a Cu–graphite electrode during the gas-phase CO2 reduction investigated by APXPS
Abstract
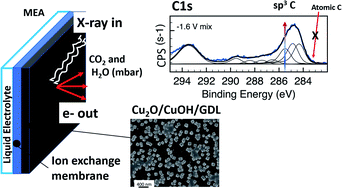
The electrocatalytic conversion of CO2 to fuels and chemicals using renewable energy is a key decarbonization technology. From a technological viewpoint, the realization of such process in the gas phase and at room temperature is considered advantageous as it allows one to circumvent the limited CO2 solubility in liquid electrolytes and CO2 transport across the electrical double layer. Yet, electrocatalysts’ performances reported so far are promising but not satisfactory. To inform the design of new materials, in this study, we apply ambient pressure X-ray photoelectron and absorption spectroscopies coupled with on-line gas detection via mass spectrometry to investigate in situ performance and interface chemistry of an electrodeposited Cu on graphitic carbon support under conditions of CO2 reduction. We use the ISISS beamline at the synchrotron facility BESSY II of the HZB and the electrochemical cell based on polymeric electrolyte membrane previously developed. We show that under cathodic potential in which methanol is formed, a fraction of the electrode with a predominantly Cu(I) electronic structure undergoes reduction to metallic Cu. The C speciation is characterized by C–O and sp3 CH3 species whereas no atomic C was formed under this condition. We also show the important role of water in the formation of methanol from accumulated surface CH3 species.
- This article is part of the themed collection: Photoelectron spectroscopy and the future of surface analysis


 Please wait while we load your content...
Please wait while we load your content...