High-efficiency electrosynthesis of urea over bacterial cellulose regulated Pd–Cu bimetallic catalyst†
Abstract
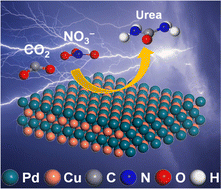
Ambient synthesis of urea through electrocatalytic coupling reaction of carbon dioxide (CO2) with nitrate (NO3−) has been regarded as a promising means to substitute the industrial energy- and capital-concentrated Haber–Bosch and Bosch–Meiser processes. Here we report the fabrication of PdCu alloying nanoparticles (NPs) anchored on carbonized bacterial cellulose (CBC) (PdCu/CBC) for ambient electrosynthesis of urea. As the electrocatalyst, the PdCu/CBC exhibits superior electrocatalytic activity toward urea synthesis with CO2 and NO3−, affording a remarkable urea yield rate of 763.8 ± 42.8 μg h−1 mgcat.−1 at −0.50 V (vs. RHE) and an exceptional faradaic efficiency (FE) of 69.1 ± 3.8% at −0.40 V (vs. RHE) under ambient conditions. The theoretical calculations unveil that this alloying catalyst provides Pd and Cu dual active sites with favored internal electron transferability, enabling generation of key *NO2 and *CO2 intermediates to facilitate C–N coupling reaction for urea synthesis. The operando spectroscopy characterization studies support the theoretical calculation results. To ensure the accuracy of the analysis data in this work, we investigated in detail the influence of the by-products in urea synthesis on the diacetyl monoxime colorimetric method and 1H nuclear magnetic resonance method for urea quantitative determination.
- This article is part of the themed collection: EES Family journals: showcase collection


 Please wait while we load your content...
Please wait while we load your content...