Oxidative sorption of arsenite from water by iron: a mechanistic perspective†
Abstract
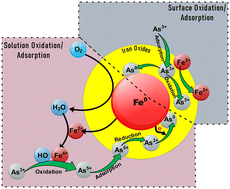
Sustenance of life without potable water is unquestionable among all communities inhabiting the earth. Reports of the past few decades showcase the alarming contamination levels of arsenic in groundwater arising from both natural and anthropogenic sources, where the complete removal is still a topic of debate. Arsenite (As(III)) is considered a more potent form of arsenic as it predominantly exists as a neutral species in natural water (pH of 6–9) making the removal process challenging. However, under the same pH conditions, the oxidized form, arsenate (As(V)), exists as a negatively charged species, allowing removal via adsorption, precipitation, or coagulation processes. This fundamental property of arsenic necessitates the conversion of arsenite to arsenate by various oxidation techniques to enhance removal from contaminated waters. To this end, iron and iron-related compounds have gained immense attention as oxidizing agents in the water treatment industry due to their natural abundance and reactivity with arsenic. This review is devoted to the discussion of the mechanisms pertaining to various iron and iron-based compounds during the oxidative adsorption of arsenite. Moreover, the review provides insight into the different technologies that can utilize iron and iron-based minerals for the elimination of arsenic from water.


 Please wait while we load your content...
Please wait while we load your content...