Novel insights into chlorine dioxide based disinfection mechanisms – investigation of the reaction with amino acids†
Abstract
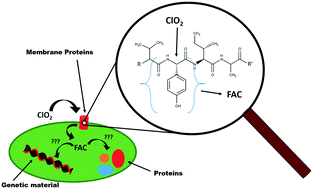
This study systematically investigated the reactions of N-acetyl-L-tyrosine (NAL-tyrosine) and N-acetyl-L-tryptophan (NAL-tryptophan) with ClO2 and FAC. NAL-tyrosine and NAL-tryptophan are examples of reactive amino acids included in peptides and incorporated in microbial membrane proteins, which can react with these oxidants during chemical water disinfection. The pH dependent reaction kinetics revealed the following order at pH 7: NAL-tyrosine + ClO2 (k = 3.16 × 104 M−1 s−1) > NAL-tryptophan + ClO2 (k = 1.81 × 104 M−1 s−1) > NAL-tryptophan + FAC (k = 7.31 × 103 M−1 s−1) ⋙ NAL-tyrosine + FAC (k = 22.6 M−1 s−1). Furthermore, this study showed that in the reactions of NAL-tyrosine and NAL-tryptophan with ClO2, free available chlorine (FAC) can form: NAL-tyrosine (50% per ClO2 consumed) and NAL-tryptophan (36% per ClO2 consumed). These results are in accordance with phenol and indole, which are the reactive moieties in NAL-tyrosine (phenolic moiety) and NAL-tryptophan (indole-moiety). Based on the achieved results, it can be assumed that in ClO2 based disinfection FAC may be an important secondary oxidant significantly contributing to the inactivation of pathogens by reacting with other cell compartments.


 Please wait while we load your content...
Please wait while we load your content...