Tracing the degradation pathway of temephos pesticide achieved with photocatalytic ZnO nanostructured films
Abstract
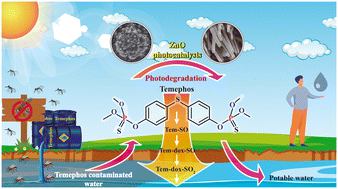
Temephos (Tem) pesticide is a permitted and widely used compound to control vector-borne diseases. Unfortunately, temephos easily undergoes fast photooxidation under ambient conditions, forming more toxic subproducts, which may cause DNA damage with its prolonged use. Semiconductor photocatalysis has proven to be an effective method to degrade toxic pollutants (including pesticides) in water, with zinc oxide (ZnO) being one of the most representative photocatalysts. In this work, the evolution of Tem and three of its principal subproducts (Tem-SO, Tem-dox-SO, and Tem-dox-SO2) was monitored using high-performance liquid chromatography (HPLC) during a simulated-sunlight photocatalytic treatment using two different ZnO nanostructured morphologies, flower-like (FL) and nanowire (NW) films. ZnO films with NW morphology were three times faster than FL in degrading Tem. NW also degraded Tem subproducts efficiently, achieving a high mineralization percentage. Their large surface area, point defects, and the contribution of Au nanoparticles on the tip of the NWs that possibly produced a surface plasmon resonance effect, enhanced visible light absorption and charge separation. These characteristics all together led to a higher production of hydroxyl radicals responsible for the degradation and mineralization of Tem and Tem subproducts. The monitoring of Tem derivatives indicated that the degradation process occurs in three stages.


 Please wait while we load your content...
Please wait while we load your content...