The influence of hardness at varying pH on zinc toxicity and lability to a freshwater microalga, Chlorella sp.†
Abstract
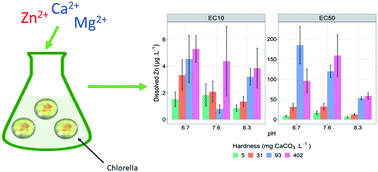
Zinc is an essential element for aquatic organisms, however, activities such as mining and refining, as well as zinc's ubiquitous role in modern society can contribute to elevated environmental concentrations of zinc. Water hardness is widely accepted as an important toxicity modifying factor for metals in aquatic systems, though other factors such as pH are also important. This study investigated the influence of increasing water hardness, at three different pH values (6.7, 7.6 and 8.3), on the chronic toxicity of zinc to the growth rate of a microalgae, Chlorella sp. Zinc toxicity decreased with increasing hardness from 5 to 93 mg CaCO3 L−1 at all three pH values tested. The 72 h growth rate inhibition EC50 values ranged from 6.2 μg Zn L−1 (at 5 mg CaCO3 L−1, pH 8.3) to 184 μg Zn L−1 (at 92 mg CaCO3 L−1, pH 6.7). Increases in hardness from 93 to 402 mg CaCO3 L−1 generally resulted in no significant (p > 0.05) reduction in zinc toxicity. DGT-labile zinc measurements did not correspond with the observed changes in zinc toxicity as hardness was varied within a pH treatment. This suggests that cationic competition from increased hardness is decreasing zinc toxicity, rather than changes in metal lability. This study highlighted that current hardness algorithms used in water quality guidelines may not be sufficiently protective of sensitive species, such as Chlorella sp., in high hardness waters.
- This article is part of the themed collection: Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...