Fully-integrated electrochemical system that captures CO2 from flue gas to produce value-added chemicals at ambient conditions†
Abstract
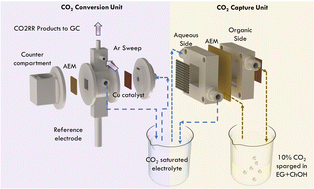
Stabilizing atmospheric CO2 calls for a significant reduction in anthropogenic CO2 emissions. There have been extensive efforts to develop individual technologies for CO2 capture and CO2 utilization (or reduction) to curb CO2 emissions efficiently. However, for rapid and economically viable reduction of atmospheric CO2, it is necessary to develop integrated technologies that capture and reduce CO2 to value-added products and fuels in a closed carbon cycle. Here, we report a systematic protocol to develop and individually evaluate electrochemical CO2 capture and electrochemical CO2 reduction processes and demonstrate a functional, fully integrated, continuous process for CO2 capture from simulated flue gas and its reduction to value-added products and fuels. For CO2 capture, we integrated a migration-assisted moisture-gradient (MAMG) CO2 capture process, where gaseous CO2 is captured as HCO3− in a CO2-binding organic liquid, transported under an electric field across an anion exchange membrane to an aqueous solution, and converted to dissolved CO2 in the presence of water-abundant environment by equilibration between HCO3−, CO2(aq), and CO32−. For CO2 reduction, we develop an electrochemical cell configuration for a CO2-free extraction of CO2 reduction gaseous products such as CO, CH4, and C2H4 on a Cu mesh catalyst. Efficient integration of the CO2 capture and CO2 reduction processes is realized based on pH-driven operating lines where the rate of CO2 capture equals the rate of CO2 reduction. We demonstrate the successful integration of these continuous CO2 captures and reduction processes to value-added products by operating the MAMG CO2 capture unit at a current of 600 mA and the CO2 reduction unit at a current density of 200 mA cm−2 resulting in 40% faradaic efficiency (FE) towards C2H4 and CO2RR FE of >57% for a simulated flue gas feed.


 Please wait while we load your content...
Please wait while we load your content...