A quasi-intercalation reaction for fast sulfur redox kinetics in solid-state lithium–sulfur batteries†
Abstract
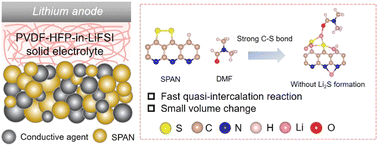
Solid-state lithium–sulfur (Li–S) batteries have been recognized as a competitive candidate for next-generation energy storage systems due to their high energy density and safety. However, the slow redox kinetics between S and Li2S and the large volume change of sulfur during charge/discharge have hindered the development of solid-state Li–S batteries. We report a solid-state Li–S battery using a polymer-in-salt solid-state electrolyte, in which the sulfur is anchored in a polyacrylonitrile (PAN) substrate during cycling, avoiding the formation of Li2S and thus resulting in much faster redox kinetics and a smaller volume change than the conventional solid-state Li–S batteries. The quasi-intercalation reaction in the system is achieved with the assistance of the residual N,N-dimethylformamide (DMF), which helps strengthen the C–S bond. As a result, the solid-state Li-sulfurized PAN (SPAN) batteries have a superb rate capability at room temperature, even higher than those of liquid-state Li–S batteries, due to the faster redox kinetics and smaller volume change without solid–solid S to Li2S conversion which is present in liquid-state Li–SPAN batteries. This is the first report of the redox kinetics of solid-state Li–SPAN batteries being increased by changing the bond-strength of the C–S bond instead of using catalysts. This technique opens up new opportunities for designing high-performance solid-state Li–S batteries.


 Please wait while we load your content...
Please wait while we load your content...