A high-rate and high-efficiency molten-salt sodium–oxygen battery†
Abstract
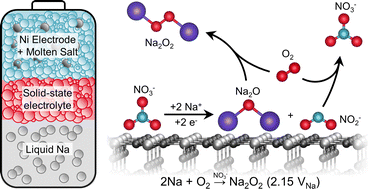
Alkali metal–oxygen batteries can provide greater specific energy than Li-ion batteries but often suffer from low power density, cycleability, and energy efficiency due to the sluggish kinetics of the oxygen electrode and parasitic reactions at both the oxygen and alkali metal electrodes. In this study, we demonstrated a molten-salt Na–O2 battery operating at 443 K with high areal energy (33 mW h cm−2geo) and power densities (19 mW cm−2geo), with high energy efficiency (∼90% at 5 mA cm−2geo), and stable cycling (400 cycles with no capacity loss). Raman, pressure tracking and titration measurements were used to show that the dominant discharge product is Na2O2. Moreover, the redox activity of nitrate anions in the molten salt was found to be critical to enable the formation of Na2O2. Through 18O-labeling experiments as well as discharging Na–Ar cells, we demonstrated that the discharge reaction occurs via the electrochemical reduction of NaNO3 to Na2O and NaNO2, where chemical reactions with O2 lead to the formation of Na2O2 from Na2O, and the regeneration of NaNO3 from NaNO2.


 Please wait while we load your content...
Please wait while we load your content...