Catalytic limitations on alkane dehydrogenation under H2 deficient conditions relevant to membrane reactors†
Abstract
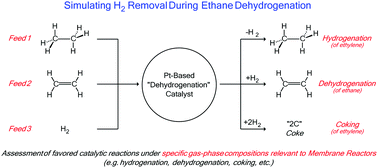
Ethane dehydrogenation, a reaction that converts ethane to ethylene and H2, is an equilibrium limited reaction, typically practiced at high temperature and low pressure in order to favor higher ethylene yields. Membrane reactors have been developed to remove H2 through the membrane walls, thereby changing the thermodynamically achievable ethylene yields as a function of H2 removal, possibly allowing for lower temperature and higher pressure reactor conditions to be realized. This work focuses on understanding the impact that these new process conditions, and specifically the new gas-phase compositions (i.e. higher ethylene and lower H2 partial pressure), have on dehydrogenation catalysts. Assessment of the impact on catalysis was carried out by feeding selected gas-phase compositions expected in a membrane reactor, by using either batch or fixed-bed units with ethane/ethylene/H2 co-feeds. Thus, by using selected co-feeds, we can simulate any conversion and any H2 removal level that may be achievable in a membrane reactor. The experiments were carried out using a highly active and selective dehydrogenation catalyst, PtSn-K/MFI. The results demonstrate that equilibrium conversion for ethane dehydrogenation is achievable at 600 °C and 1 atm on PtSn–K/MFI. However, upon simulating H2-removal conditions, the new equilibrium conversions were not achievable. The inability to obtain the new equilibrium conversions at simulated H2 removal is due to, in part, deleterious reactions, most notably coking. Coking reactions lead to deactivation of the catalyst, and these coking reactions are accelerated by H2 removal. Moreover, prior to significant catalyst deactivation, the dehydrogenation reactions that lead to coke produce additional H2, which further limit ethylene yields due to re-hydrogenation of ethylene. Thus, while thermodynamics predicts higher olefin yields due to H2 removal based solely on the ethane dehydrogenation reaction pathway, the substantial effect the process conditions have on catalysis and coking reactions limits achievable yields, and must be accounted for when assessing H2 removal and membrane technology.


 Please wait while we load your content...
Please wait while we load your content...