A universal strategy for high-voltage aqueous batteries via lone pair electrons as the hydrogen bond-breaker†
Abstract
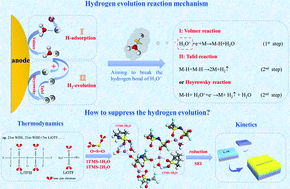
Aqueous batteries have attracted extensive attention for their safety, low cost, and non-toxicity properties. However, the narrow electrochemical stability window and freezing of water at a low temperature limit the energy density and working temperature range of aqueous Li-ion batteries. Herein, we introduce a “hydrogen bond-captured” solvent, which has lone pair electrons on the oxygen atom, to break the original water hydrogen bond network by forming intermolecular hydrogen bonds, resulting in the suppressed hydrogen evolution reaction (HER) and reduced water activity. The “LiTFSI(TMS)0.5Water” electrolyte enables stable interfacial chemistry, broadens the electrochemical stability window to 5.4 V and exhibits a freezing point lower than −85 °C. An aqueous LiNi0.5Mn1.5O4/Li4Ti5O12 full cell achieved an energy density of 136 W h kg−1 for 300 cycles at 6C. An understanding of how to alter the thermodynamics pathway of the HER in the electrolyte provides a practical guideline for designing high-voltage and wide temperature range aqueous electrolytes for sustainable energy storage applications.


 Please wait while we load your content...
Please wait while we load your content...