Membrane-less amphoteric decoupled water electrolysis using WO3 and Ni(OH)2 auxiliary electrodes†
Abstract
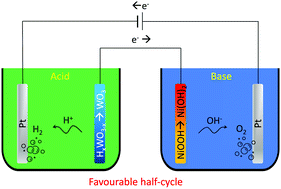
Energy storage and delivery play a crucial role in the effective management of renewable power sources such as solar and wind. Hydrogen energy is proposed to be one of the major substitutes to fill the gap between the production plant and consumer. The energy from renewable power sources is used to generate hydrogen, which is later converted to electricity and water. Hydrogen generation in water electrolysis from renewable energy is a sustainable process. However, the need for membrane separation of hydrogen from oxygen in single-cell water electrolysis is detrimental. Moreover, the hydrogen production rate in conventional single-cell electrolysers is strictly limited by the rate of oxygen evolution. Recently decoupled water electrolysis has been proposed where hydrogen and oxygen are generated in spatially separated alkaline cells. Here we demonstrate amphoteric decoupled electrolysis by using an auxiliary electrode (AE) couple with HxWO3 and NiOOH being employed in separate acid and alkaline cells, respectively. The average electrolysis efficiency of the proposed concept is up to 71%, higher than that observed from decoupled electrolysis where both cells are alkaline.


 Please wait while we load your content...
Please wait while we load your content...