Direct conversion of CO2 to solid carbon by Ga-based liquid metals†
Abstract
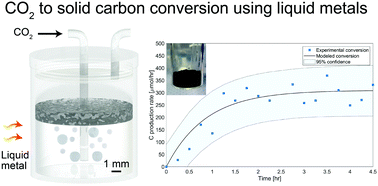
The direct conversion of CO2 to carbon is a highly providential route; however, conventional thermal and catalytic approaches are hindered by high energy demands and are limited by coking. Here, we report a robust and highly selective method for the direct conversion of CO2 to solid carbon over EGaIn liquid metal (LM) alloy. We utilized the low-melting point of this alloy to facilitate the reduction of CO2 at low temperatures, producing 319 μmol h−1 of carbon at 200 °C, and remarkably enabling CO2 activation and carbon production even at room temperature, without the use of a supplementary reductant such as hydrogen. The deployed LM showed no signs of deactivation by coking and the generated carbon is shown to naturally accumulate at the top of the LM where it can be easily collected. In situ XPS measurements indicate an increase of 9.6% in the carbon–carbon bond content and an equivalent decrease in the Ga metal content, upon exposure of the LM to CO2 for 30 mins at 200 °C and 1 bar. This led to the conclusion that solid carbon and gallium oxide are the final reaction products of this process. Density functional theory calculations shed further light on the adsorption and dissociation of CO2 over Ga and EGaIn. The presented method creates a pathway to transforming CO2 to perpetually stored solid carbon and can therefore set a trajectory for making a measurable impact on carbon intensive industries.


 Please wait while we load your content...
Please wait while we load your content...