Molecular-level insight into uptake of dimethylamine on hydrated nitric acid clusters†
Abstract
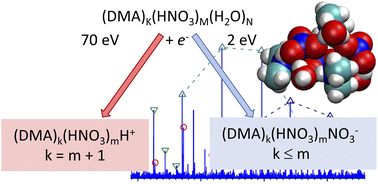
Mixed nitric acid/water clusters with dimethylamine (DMA) represent a suitable model system for understanding acid–base chemistry in atmospherically relevant clusters. We investigate these clusters in a detailed molecular-beam experiment accompanied by ab initio calculations. The (HNO3)M(H2O)N clusters are produced by supersonic expansion into vacuum and doped by DMA molecules in a pickup process. Two complementary mass spectrometry approaches are employed to analyze the resulting (DMA)K(HNO3)M(H2O)N clusters: (i) electron impact ionization at 70 eV to form positive cluster ions and (ii) low-energy electron attachment at 0–10 eV to form negative clusters. The positive ion spectra contain mainly protonated (DMA)k(HNO3)mH+ clusters with k = m + 1, whereas the negative ones are dominated by (DMA)k(HNO3)mNO−3 with m ≥ k, followed by (DMA)k(HNO3)mNO2− (m > k) ions with low abundances. These observations are rationalized by our calculations, which exhibit the tendency of the mixed clusters to maximize the number DMA·H+ and NO3− ions in the clusters. In the neutral clusters, this is fulfilled for 1 : 1 ratio of DMA and HNO3, while the positively charged (DMA)k(HNO3)mH+ clusters satisfy this condition for k = m + 1. The protonated clusters always contain the DMA·H+ moiety. For the negatively charged cluster ions, thermochemistry supports the prevailing formation of NO−3 and m ≥ k ion composition. Furthermore, the NO−3-containing cluster ions can form when an electron attaches to the protonated moiety of the DMA·H+⋯NO−3 ion pair in the cluster, which leads to H atom evaporation. From the gas phase HNO3 molecule, where NO−2 is formed exclusively upon an electron attachment, the tendency to form NO−3 increases to hydrated HNO3 clusters, where both NO−2 and NO−3 ions are generated in approximately equal abundances, to the DMA doped clusters, where NO−3 strongly prevails NO−2.


 Please wait while we load your content...
Please wait while we load your content...