Anticancer ruthenium(ii) tris(pyrazolyl)methane complexes with bioactive co-ligands†
Abstract
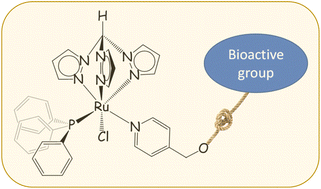
In comparison with RuII-arene compounds, the medicinal potential of homologous RuII-tpm compounds [tpm = tris(pyrazolyl)methane] is underexplored. Pyridine, 4-pyridinemethanol and four functionalized pyridines, synthesized from the esterification of 4-pyridinemethanol with bioactive carboxylic acids (i.e., ethacrynic acid, ibuprofen, flurbiprofen and naproxen), react with the precursor [RuCl(κ3-tpm)(PPh3)2]Cl (1) to afford [RuCl(κ3-tpm)(PPh3)(L)]Cl (2–7, L = pyridine ligand), in 78–91% yields. All products were fully characterized by HR-ESI mass spectrometry, IR and multinuclear NMR spectroscopy and the solid-state structures of two of the complexes, i.e. where L = pyridine and 4-pyridinemethanol, were ascertained by single crystal X-ray diffraction. The {Ru-tpm-PPh3} assembly is stable in D2O and in biological medium (DMEM) at 37 °C, with a tendency to slowly dissociate the pyridine ligand. The antiproliferative activity of the complexes was assessed on the cancerous A2780 and A2780cisR cell lines, and the nontumoral HEK 293T cell line; moreover inhibition assays were carried out on the complexes towards COX-2 and GSTP1 enzymes.


 Please wait while we load your content...
Please wait while we load your content...