Preparation of new microporous europium silicate molecular sieve by selective leaching of alkali metal cations from europium silicate Eu-AV-9†
Abstract
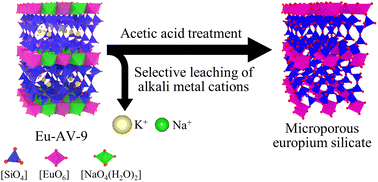
Acid treatment of crystalline silicates is a facile method of creating pores for the preparation of crystalline silica-based microporous materials, but its success depends on the acid treatment conditions and both the composition and crystallinity of the starting silicates. Here, europium silicate Eu-AV-9 containing Na+, K+, and Eu3+ ions was treated with acetic acid for 1 d or 14 d to synthesize microporous silicate with high Eu loading by the selective leaching of K+ and Na+ from the silicate. The acid-treated Eu-AV-9 had both crystallinity and microporosity capable of adsorbing CO2, while the adsorption of Ar was very low. In addition, the values of both micropore volume and BET area of acid-treated samples increased when the time of acid treatment was increased. This result was attributed to the formation of structural defects caused by eliminating Eu in Eu-AV-9 over time of acid treatment.


 Please wait while we load your content...
Please wait while we load your content...