Elucidating the mechanism of photochemical CO2 reduction to CO using a cyanide-bridged di-manganese complex†
Abstract
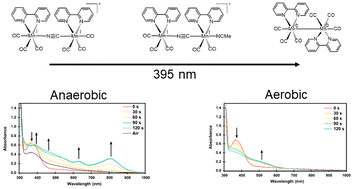
The complex, [{[Mn(bpy)(CO)3]2}(μ-CN)]+ (Mn2CN+), has previously been shown to photochemically reduce CO2 to CO. The detailed mechanism behind its reactivity was not elucidated. Herein, the photoevolution of this reaction is studied in acetonitrile (MeCN) using IR and UV-vis spectroscopy. Samples were excited into the MnI → π* bpy metal-to-ligand charge transfer (MLCT) absorption band triggering CO loss, and rapid MeCN solvent ligation at the open coordination site. It is concluded that this process occurs selectively at the Mn axial ligation site that is trans to the C-end of the bridging cyanide. Upon further photolysis, the metal–metal bonded dimeric species, [(CO)3(bpy)Mn–Mn(bpy)(CO)3] (Mn–Mn) is observed to form under anaerobic conditions. The presence of this dimeric species coincides with the observation of CO production. When oxygen is present, CO2 photoreduction does not occur, which is attributed to the inability of Mn2CN+ to convert to the metal–metal bonded dimer. Photolysis experiments, where the Mn–Mn dimer is formed photochemically under argon first and then exposed to CO2, reveal that it is the radical species, [Mn(bpy)(CO)3˙] (Mn˙), that interacts with the CO2. Since the presence of Mn–Mn and light is required for CO production, [Mn(bpy)(CO)3˙] is proposed to be a photochemical reagent for the transformation of CO2 to CO.


 Please wait while we load your content...
Please wait while we load your content...