Investigating the chemical sensitivity of melting in zeolitic imidazolate frameworks†
Abstract
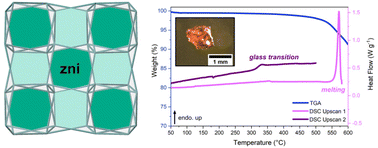
The number of zeolitic imidazolate frameworks (ZIFs) that form melt-quenched glasses remains limited, with most displaying the cag network topology. Here, we expand our studies to zni topology ZIFs, starting with ZIF-zni [Zn(Im)2] before changing its linker chemistry, by incorporating 2-methylimidazolate and 5-aminobenzimidazolate. ZIF-zni was found to melt and form a glass, with Tm = 576 °C and Tg = 322 °C, although it was not possible to prepare the glass without zinc oxide impurities. The addition of 2-methylimidazolate to the structure gave ZIF-61 [Zn(Im)1.35(mIm)0.65], which decomposed without passing through the liquid state. However, incorporating small quantities of 5-aminobenzimidazolate resulted in a ZIF [Zn(Im)1.995(abIm)0.005] with a lower melting temperature (Tm = 569 °C) than pure ZIF-zni, and no evidence of zinc oxide growth. This demonstrates the sensitivity of melting behaviour in ZIFs towards linker chemistry, with only a 0.25% variation capable of eliciting a 7 °C change in melting temperature. This study highlights the chemical sensitivity of melting in ZIFs and serves as a promising strategy for tuning their melting behaviour.


 Please wait while we load your content...
Please wait while we load your content...