BODIPY and dipyrrin as unexpected robust anchoring groups on TiO2 nanoparticles†
Abstract
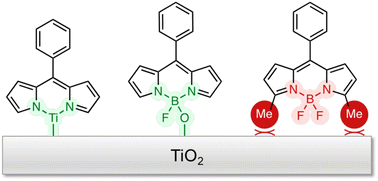
Covalent attachment of molecules to metal oxide surfaces typically demands the presence of an anchoring group that in turn requires synthetic steps to introduce. BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) chromophores have long been used in dye-sensitized solar cells, but carboxylic acid groups typically had to be installed to act as surface anchors. We now find that even without the introduction of such anchors, the unmodified BODIPY can bind to TiO2 surfaces via its BF2 group through boron–oxygen surface bonds. Dipyrrin, the parent molecule of BODIPY, is also capable of binding directly to TiO2 surfaces, likely through its chelating nitrogen atoms. These binding modes prove to be even more robust than that of an installed carboxylate and offer a new way to attach molecular complexes to surfaces for (photo)catalytic applications since, once bound, we show that surface bound BODIPY and dipyrrin derivatives exhibit ultrafast photoinjection of electrons into the conduction band of TiO2.


 Please wait while we load your content...
Please wait while we load your content...