Synthesis, characterisation and reactivity of group 2 complexes with a thiopyridyl scorpionate ligand†
Abstract
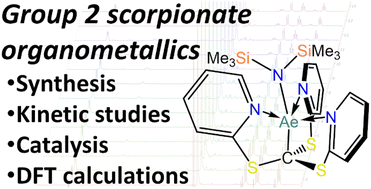
Herein we report the reactivity of the proligand tris(2-pyridylthio)methane (HTptm) with various Alkaline Earth (AE) reagents: (1) dialkylmagnesium reagents and (2) AE bis-amides (AE = Mg–Ba). Heteroleptic complexes of general formulae [Mg(Tptm)(R)] (R = Me, nBu; Tptm = {C(S–C5H4N)3}−) and [AE(Tptm)(N′′)] (AE = Mg–Ba; N′′ = {N(SiMe3)2}−) were targeted from the reaction of HTptm with R2Mg or [AE(N′′)2]2. Reaction of the proligand with dialkylmagnesium reagents led to formation of [{Mg(κ3C,N,N–C{Bu}{S–C5H4N}2)(μ-S–C5H4N)}2] (1) and [{Mg(κ3C,N,N–C{Me}{S–C5H4N}2)(μ-OSiMe3)}2] (2) respectively, as a result of a novel transfer of an alkyl group onto the methanide carbon with concomitant C–S bond cleavage. However, reactivity of bis-amide precursors for Mg and Ca did afford the target species [AE(Tptm)(N′′)] (3-AE; AE = Mg–Ca), although these proved susceptible to ligand degradation processes. DFT calculations show that alkyl transfer in the putative [Mg(Tptm)(nBu)] (1m′) system and amide transfer in 3-Ca is a facile process that induces C–S bond cleavage in the Tptm ligand. 3-Mg and 3-Ca were also tested as catalysts for the hydrophosphination of selected alkenes and alkynes, including the first example of mono-hydrophosphination of 4-ethynylpyridine which was achieved with high conversions and excellent regio- and stereochemical control.


 Please wait while we load your content...
Please wait while we load your content...