Synthesis and structural characterization of the new Zintl phases Eu10Mn6Bi12 and Yb10Zn6Sb12†
Abstract
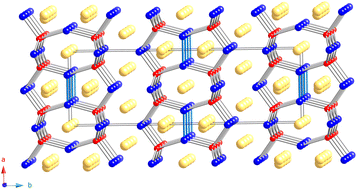
Two new ternary compounds, Eu10Mn6Bi12 and Yb10Zn6Sb12, were synthesized and structurally characterized. The synthesis was achieved either through reactions in sealed niobium tubes or in alumina crucibles by combining the elements in excess molten Sb. Their structures were elucidated using single-crystal X-ray diffraction, and they were determined to crystallize in the orthorhombic space group Cmmm (no. 65) with the Eu10Cd6Bi12 structure type. Akin to the archetype phase, both Mn and Zn sites contain about 25% of vacancies. The anionic substructure of the title phases can be described as [M6Pn12] (M = Zn, Mn; Pn = Sb, Bi) double layers composed of the corner and edge-sharing [MPn4] tetrahedra, linked by [Pn2]4− dumbbells. Eu2+/Yb2+ cations fill the space between the layers, with the valence electron counts adhering closely to the Zintl–Klemm rules, i.e., both Eu10Mn6Bi12 and Yb10Zn6Sb12 are expected to be valence-precise compounds. Analysis of the electronic structure and transport properties of Yb10Zn6Sb12 indicate semimetallic behavior with relatively low Seebeck coefficient and resistivity that slightly decreases as a function of temperature.


 Please wait while we load your content...
Please wait while we load your content...