Synthesis and characterization of a new monometallic layered double hydroxide using manganese†
Abstract
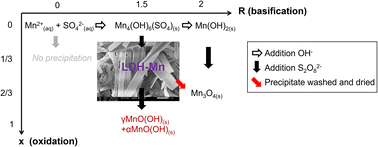
This article reports for the first time the synthesis of an LDH using only manganese as the divalent and trivalent metallic ion. Analysis of the pH, redox potential, and chemical composition during the oxidation of a manganese basic salt using persulfate indicates the oxidation of 1/3 of the initial MnII ions, in agreement with the paramagnetic structure and XPS analysis. Infrared, Raman spectra and thermogravimetric analysis results were similar to the ones obtained with Fe-LDH also known as green rust. X-Ray diffractograms and Rietveld refinement were used to determine the structure of this solid. Thermodynamic considerations predict that this solid could reduce nitrate into gaseous nitrogen without further reduction to ammonium or ammonia unlike what is observed for Fe-LDH.


 Please wait while we load your content...
Please wait while we load your content...