Benzothiazole-substituted 1,3-diaza-2-oxophenoxazine as a luminescent nucleobase surrogate for silver(i)-mediated base pairing†
Abstract
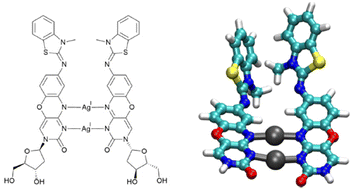
A benzothiazole-substituted derivative (X) of 1,3-diaza-2-oxophenoxazine was evaluated with respect to its ability to engage in Ag(I)-mediated homo base pair formation in two different DNA duplexes. The metal binding was determined by a combination of temperature-dependent UV spectroscopy, CD spectroscopy, and fluorescence spectroscopy, indicating the incorporation of two Ag(I) ions to generate a dinuclear X–Ag(I)2–X base pair. Interestingly, a luminescence increase was observed upon metal binding. Theoretical luminescence spectra were calculated using time-dependent density functional theory (TDDFT) for all possible Ag(I)-mediated X : X base pair geometries to identify the species responsible for the increase in luminescence. The study shows that even bulky non-planar artificial nucleobases can be applied to form stabilizing metal-mediated base pairs.


 Please wait while we load your content...
Please wait while we load your content...