Covalent triflates as synthons for silolyl- and germolyl cations†
Abstract
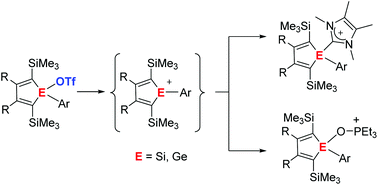
The synthesis of 1-silolyl and 1-germolyl triflates from the corresponding chlorides by salt metathesis reaction is reported. These covalent triflates are ideal starting materials for the preparation of ionic silolyl- and germolyl-imidazolium triflates by their reaction with N-heterocyclic carbenes. Similarily, ionic silolyl- and germolyl-oxophosphonium triflates are obtained by substitution of the triflate group by triethylphosphane oxide Et3PO. The analysis of their 31P NMR chemical shifts according to the Gutmann–Beckett method reveal the high Lewis acidity of the underlying silolyl and germolyl cations. Further analysis of structural and NMR parameters of the silolyl- and germolyl-imidazolium and oxophosphonium triflates indicates that these compounds are covalently bonded silole and germole derivatives with insignificant contributions from silolyl- or germolyl cations. Silolyl and germolyl triflates are however synthetic equivalents of these cations and might serve as a source for electrophilic silolyl and germolyl units.


 Please wait while we load your content...
Please wait while we load your content...