Ruthenium(ii) complexes with phosphonate-substituted phenanthroline ligands: synthesis, characterization and use in organic photocatalysis†
Abstract
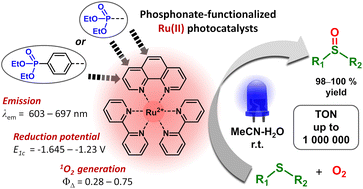
Ru(II) complexes with polypyridyl ligands play a central role in the development of photocatalytic organic reactions. This work is aimed at the structural modification of such complexes to increase their photocatalytic efficiency and adapt them for the preparation of reusable photocatalytic systems. Nine [Ru(phen)(bpy)2]2+-type complexes (bpy = 2,2′-bipyridine, phen = 1,10-phenanthroline) (Ru-Pcat) bearing the P(O)(OEt)2 substituent attached to the phen core directly or through a 1,4-phenylene linker were synthesized and characterized by spectroscopic and electrochemical techniques. The coordination mode of phen ligands was confirmed by single crystal X-ray analysis. The (spectro)electrochemical data show that the first electron transfer in Ru-Pcat takes place on the phen ligand. The emission maxima and quantum yields are strongly affected by the substitution pattern, reaching the far-red region (697 nm) for Ru-3,8P2. The singlet oxygen quantum yields of Ru-Pcat were evaluated using the chemical trapping method. Finally, the photocatalytic performance of Ru-Pcat in the oxidation of sulfides with molecular oxygen was investigated. Both dialkyl and alkyl aryl sulfides were quantitatively transformed into sulfoxides under irradiation with a blue LED in the acetonitrile–water mixture (10 : 1) using a low loading of 0.005–0.05 mol% Ru(II) photocatalysts. To rationalize the effect of phosphonate substituents on the photocatalytic efficiency, comparative kinetic studies of (1) 4-nitrothioanisole oxidation proceeding predominantly via the electron transfer pathway and (2) oxidation of dibutyl sulfide wherein singlet oxygen serves as an oxidant have been performed. It was demonstrated that complexes with the P(O)(OEt)2 substituent at positions 4 and 7 outperform the benchmark photocatalyst Ru-(bpy)3 and the parent complex Ru-phen in the reactions proceeding through electron transfer (reductive quenching photocatalytic cycle). The TON in the oxidation of 4-methoxythioanisole was found to be as high as 1 000 000 that is, to our knowledge, the highest among previously reported photocatalysts. In contrast, upon separating the P(O)(OEt)2 group and the phen core with the 1,4-phenylene linker, singlet oxygen quantum yields significantly increase that favors reactions proceeding through energy transfer (the oxidation of dibutyl sulfide in our case). Thus, both series of Ru(II) complexes prepared in this work are promising for the improvement of known photocatalytic reactions and the development of new transformations.


 Please wait while we load your content...
Please wait while we load your content...