Synthesis and structural properties of a 2D Zn(ii) dodecahydroxy-closo-dodecaborate coordination polymer†
Abstract
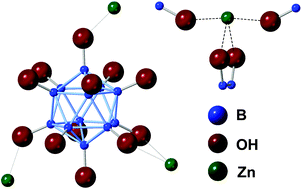
In this work, we discuss the synthesis and characterization of a 2D coordination polymer composed of a dianionic perhydroxylated boron cluster, [B12(OH)122−], coordinated to Zn(II)—the first example of a transition metal-coordinated [B12(OH)12]2− compound. This material was synthesized via cation exchange from the starting cesium salt and then subjected to rigorous characterization prior to and after thermal activation. Numerous techniques, including XRD, FTIR, SEM, TGA, and solid-state NMR revealed a 2D coordination polymer composed of sheets of Zn(II) ions intercalated between planes of boron clusters. The as-synthesized material was then evacuated of solvent via thermal treatment, and atomic-level changes from this transformation were elucidated through a combination of 1D and 2D solid-state NMR analyses of 11B and 1H nuclei, suggesting the full removal of coordinated solvent molecules. Evidence also suggested that [B12(OH)122−] can adjust its coordination to Zn(II) in the solid-state through hemilability of its numerous –OH ligands.


 Please wait while we load your content...
Please wait while we load your content...