Spectroscopic and electrochemical characterization of a Pr4+ imidophosphorane complex and the redox chemistry of Nd3+ and Dy3+ complexes†
Abstract
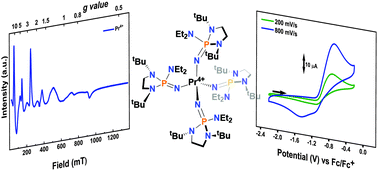
The molecular tetravalent oxidation state for praseodymium is observed in solution via oxidation of the anionic trivalent precursor [K][Pr3+(NP(1,2-bis-tBu-diamidoethane)(NEt2))4] (1-Pr(NP*)) with AgI at −35 °C. The Pr4+ complex is characterized in solution via cyclic voltammetry, UV-vis-NIR electronic absorption spectroscopy, and EPR spectroscopy. Electrochemical analyses of [K][Ln3+(NP(1,2-bis-tBu-diamidoethane)(NEt2))4] (Ln = Nd and Dy) by cyclic voltammetry are reported and, in conjunction with theoretical modeling of electronic structure and oxidation potential, are indicative of principal ligand oxidations in contrast to the metal-centered oxidation observed for 1-Pr(NP*). The identification of a tetravalent praseodymium complex in in situ UV-vis and EPR experiments is further validated by theoretical modeling of the redox chemistry and the UV-vis spectrum. The latter study was performed by extended multistate pair-density functional theory (XMS-PDFT) and implicates a multiconfigurational ground state for the tetravalent praseodymium complex.


 Please wait while we load your content...
Please wait while we load your content...
