Use of heterometallic alkali metal–magnesium aryloxides in ring-opening polymerization of cyclic esters†
Abstract
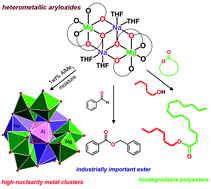
In this work, alkali metal–magnesium aryloxides [Mg2Li2(MesalO)6] (1), [Mg2Na2(MesalO)6(THF)x] for x = 2 or 4 (2), and [Mg2K2(MesalO)6(THF)4] (3) derived from the reaction of MgnBu2 and nBuLi, metallic Na or K with methyl salicylate (MesalOH) were used as molecular platforms for the synthesis of new heterometallic compounds [Mg2Li2(EtsalO)6] (4), [MgK(EtsalO)3]n (5), [Mg6Na4Al(MesalO)13(OH)6(MesalOH)(THF)0.5(H2O)0.5] (6), and [Mg4Na2(MesalO)6(SalO)2(THF)4] (7) (EtsalOH = ethyl salicylate and SalOH2 = salicylic acid) by the reaction with EtOH, exposure to atmospheric moisture or addition of stoichiometric quantities of water. Compounds 4 and 5 were synthesized by transesterification of 1 and 3. Cluster 6 was formed haphazardly by exposing a THF solution of 2 derived using MgnBu2 stabilized with 1 wt% AlEt3 to atmospheric moisture. Compound 7 was synthesized by partial hydrolysis of 2. Homometallic magnesium aryloxide [Mg4(MesalO)4(OMe)4(HOMe)4] (8) was obtained by reaction of MgnBu2 and MesalOH in a methanol solution. The catalytic activity of 1–3 and 6–8 was investigated in the ring-opening polymerization (ROP) of L-lactide (L-LA) or benzaldehyde Tishchenko reaction.


 Please wait while we load your content...
Please wait while we load your content...