Mechanistic study on reduction of nitric oxide to nitrous oxide using a dicopper complex†
Abstract
A density functional theory study was carried out to investigate the reduction mechanisms of NO to N2O using a dicopper complex reported by Zhang and coworkers (J. Am. Chem. Soc., 2019, 141, 10159–10164). The reaction mechanism consists of three steps: N–N bond formation, isomerization of the resultant N2O2 moiety, and cleavage of the N–O bond.
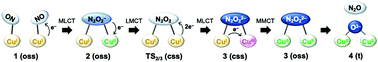


 Please wait while we load your content...
Please wait while we load your content...