The chameleon-like nature of elusive cobalt–oxygen intermediates in C–H bond activation reactions†
Abstract
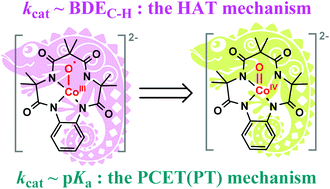
High-valence metal-oxo (M–O, M = Fe, Mn, etc.) species are well-known reaction intermediates that are responsible for a wide range of pivotal oxygenation reactions and water oxidation reactions in metalloenzymes. Although extensive efforts have been devoted to synthesizing and identifying such complexes in biomimetic studies, the structure–function relationship and related reaction mechanisms of these reaction intermediates remain elusive, especially for the cobalt–oxygen species. In the present manuscript, the calculated results demonstrate that the tetraamido macrocycle ligated cobalt complex, Co(O)(TAML) (1), behaves like a chameleon: the electronic structure varies from a cobalt(III)–oxyl species to a cobalt(IV)-oxo species when a Lewis acid Sc3+ salt coordinates or an acidic hydrocarbon attacks 1. The dichotomous correlation between the reaction rates of C–H bond activation by 1 and the bond dissociation energy (BDE) vs. the acidity (pKa) was rationalized for the first time by different reaction mechanisms: for normal C–H bond activation, the Co(III)–oxyl species directly activates the C–H bond via a hydrogen atom transfer (HAT) mechanism, whereas for acidic C–H bond activation, the Co(III)–oxyl species evolves to a Co(IV)-oxo species to increase the basicity of the oxygen to activate the acidic C–H bond, via a novel PCET(PT) mechanism (proton-coupled electron transfer with a PT(proton-transfer)-like transition state). These theoretical findings will enrich the knowledge of biomimetic metal–oxygen chemistry.


 Please wait while we load your content...
Please wait while we load your content...