Mono-copper far more active than analogous di-copper complex for electrocatalytic hydrogen evolution†
Abstract
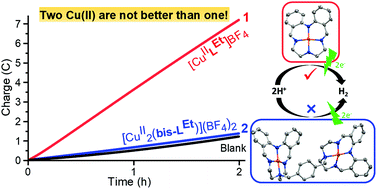
The di-copper(II) analogue, [CuII2(bis-LEt)](BF4)2 (2), of the previously reported mono-copper(II) complex [CuIILEt]BF4 (1) which resulted in long lived electrocatalytic hydrogen evolution reaction (HER), has been prepared, characterised and tested for HER. The new bis-macrocycle, bis-HLEt, was formed from two HLEt Schiff base macrocycles (prepared by 1 + 1 condensation of 2,2′-iminobisbenzaldehyde and diethylenetriamine) being connected by selective alkylation of the less sterically hindered secondary alkyl amine group (NH) of each, using α,α′-dibromo-para-xylene to form a linker between them. The desired dicopper(II) complex, [Cu2II(bis-LEt)](BF4)2·4H2O (2·4H2O), was readily prepared, as a yellowish brown solid in 82% yield. SCXRD on yellow-brown crystals of [CuII2(bis-LEt)](BF4)2·2MeCN (2·2MeCN) revealed both copper(II) centres are square planar with a very similar copper(II) coordination environment to that of square planar 1. Although dicopper(II) complex 2 is easier to reduce than the analogous monocopper(II) complex 1 in MeCN (E1/2(ΔE): 2 −1.20(0.12) V, 1 −1.39(0.09) V, vs. 0.01 M AgNO3/Ag), electrocatalytic HER testing of dicopper complex 2·4H2O, in MeCN with 80 equivalents of acetic acid, revealed it was inactive, in stark contrast to the high and ongoing activity of 1 under the same conditions. So two is definitely not better than one metal ion in this case. Rather, it may be that the presence of an NH group in the macrocycle of 1, but absent in the bis-macrocycle of 2 (due to alkylating that NH to link the two macrocycles), may be key to the HER activity seen for 1.


 Please wait while we load your content...
Please wait while we load your content...