1,2,3-Triazole based ligands with phosphine and pyridine functionalities: synthesis, PdII and PtII chemistry and catalytic studies†
Abstract
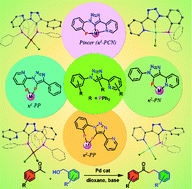
This manuscript describes the syntheses of pyridine appended triazole-based mono- and bisphosphines, [o-Ph2P(C6H4){1,2,3-N3C(Py)C(H)}] (2), [o-Br(C6H4){1,2,3-N3C(Py)C(PPh2)}] (3), [C6H5{1,2,3-N3C(Py)C(PPh2)}] (4), [Ph2P(C6H4){1,2,3-N3C(Py)C(PPh2)}] (5) and [3-Ph2P-2-{1,2,3-N3C(Ph)C(PPh2)}C5H3N] (6), their palladium and platinum chemistry and catalytic applications. These ligands upon treatment with [M(COD)Cl2] (M = Pd or Pt) yielded complexes with different coordination modes, depending on the reaction conditions. Both κ2-P,N and κ2-P,P coordination modes were observed in many of the complexes indicating the ambidentate nature of these ligands. Monophosphine 2 in the presence of a base afforded rare fused-5,6-membered PCN pincer complexes [MCl{o-Ph2P(C6H4){1,2,3-N3C(Py)C(H)}}-κ3-P,C,N] (7, M = Pd; 8, M = Pt), whereas the reactions of 4 with [M(COD)Cl2] (M = Pd, Pt) produced κ2-P,N chelate complexes [MCl2{C6H5{1,2,3-N3C(Py)C(PPh2)}-κ2-P,N}] (9, M = Pd; 10, M = Pt). Similar reactions of 5 and 6 resulted in κ2-P,P chelate complexes [MCl2{{3-Ph2P-2-{1,2,3-N3C(Ph)C(PPh2)}C5H3N}-κ2-P,P}] (11, M = Pd; 12, M = Pt) and [MCl2{3-Ph2P-2-{1,2,3-N3C(Ph)C(PPh2)}C5H3N}-κ2-P,P}] (13, M = Pd; 14, M = Pt), respectively. The palladium(II) complexes have shown excellent catalytic activity in the α-alkylation reaction of acetophenone derivatives.


 Please wait while we load your content...
Please wait while we load your content...