Complexes of Zn(ii) with a mixed tryptanthrin derivative and curcumin chelating ligands as new promising anticancer agents†
Abstract
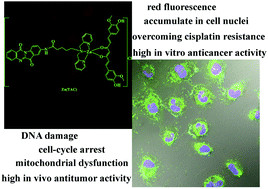
In this study, two novel curcumin (H-Cur)–tryptanthrin metal compounds—[Zn(TA)Cl2], i.e., Zn(TA), and [Zn(TA)(Cur)]Cl, i.e., Zn(TAC)—were synthesized and investigated using 5-(bis-pyridin-2-ylmethyl-amino)-pentanoic acid (6,12-dioxo-6,12-dihydro-indolo[2,1-b]quinazolin-8-yl)-amide (TA) and H-Cur as the targeting and high-activity anticancer chemotherapeutic moieties, respectively. They were then compared with the di-(2-picolyl)amine (PA) Zn(II) complex [Zn(PA)Cl2], i.e., Zn(PA). When compared with Zn(PA) and cisplatin, the IC50 values of Zn(TA) and Zn(TAC) indicated that the compounds had high cytotoxicity against A549/DDP cancer cells, implying that the H-Cur–tryptanthrin Zn(II) compounds have the potential for use as anticancer drugs. We propose the use of synthesized theragnostic H-Cur–tryptanthrin Zn(II) complexes with nuclear-targeting and DNA-damaging capabilities as a simple therapeutic strategy against tumors. The Zn(TA) and Zn(TAC) complexes could be traced via red fluorescence and were found to accumulate in the cell nuclei and induce DNA damage, cell cycle arrest, mitochondrial dysfunction, and cell apoptosis both in vitro and in vivo. In addition, Zn(TAC) exhibited a higher antiproliferative effect on A549/DDP than Zn(TA) and Zn(PA), which was undoubtedly associated with the key roles of the novel tryptanthrin derivative TA and H-Cur in the Zn(TAC) complex.


 Please wait while we load your content...
Please wait while we load your content...