Mechanism and selectivity of photocatalyzed CO2 reduction by a function-integrated Ru catalyst†
Abstract
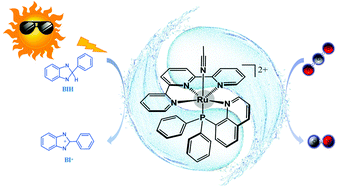
The phosphine-substituted Ru(II) polypyridyl complex, [RuII-(tpy)(pqn)(MeCN)]2+ (RuP), was disclosed to be an efficient photocatalyst for the reduction of CO2 to CO with excellent selectivity. In this work, density functional calculations were performed to elucidate the reaction mechanism and understand the origin of selectivity. The calculations showed that RuP was first excited to the singlet excited state, followed by intersystem crossing to produce a triplet species (3RuIII(L˙−)–S), which was then reduced by the sacrificial electron donor BIH to generate a RuII(L˙−) intermediate. The ligand of RuII(L˙−) was further reduced to produce a RuII(L2−) intermediate. The redox non-innocent nature of the tpy and pqn ligands endows the Ru center with an oxidation state of +2 after two one-electron reductions. RuII(L2−) nucleophilically attacks CO2, in which two electrons are delivered from the ligands to CO2, affording a RuII–COOH species after protonation. This is followed by the protonation of the hydroxyl moiety of RuII–COOH, coupled with the C–O bond cleavage, resulting in the formation of RuII–CO. Ultimately, CO is dissociated after two one-electron reductions. Protonation of RuII(L2−) to generate a RuII–hydride, a critical intermediate for the production of formate and H2, turns out to be kinetically less favorable, even though it is thermodynamically more favorable. This fact is due to the presence of a Ru2+ ion in the reduced catalyst, which disfavors its protonation.


 Please wait while we load your content...
Please wait while we load your content...