C(sp3)–H bond activation by the carboxylate-adduct of osmium tetroxide (OsO4)†
Abstract
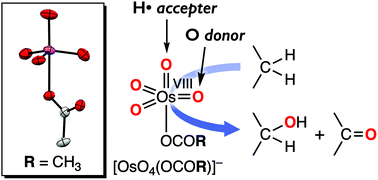
The reaction of osmium tetroxide (OsO4) and carboxylate anions (acetate: X− = AcO− and benzoate: X− = BzO−) gave 1 : 1 adducts, [OsO4(X)]− (1X), the structures of which were determined by X-ray crystallographic analysis. In both cases, the carboxylate anion X coordinates to the osmium centre to generate a distorted trigonal bipyramidal osmium(VIII) complex. The carboxylate adducts show a negative shift of the redox potentials (E1/2) and a red shift of the νOs![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretches as compared to those of tetrahedral OsO4 itself. Despite the negative shift of E1/2, the reactivity of these adduct complexes 1X was enhanced compared to that of OsO4 in benzylic C(sp3)–H bond oxidation. The reaction obeyed the first-order kinetics on both 1X and the substrates, giving the second-order rate constant (k2), which exhibits a linear correlation with the C–H bond dissociation energy (BDEC–H) of the substrates (xanthene, 9,10-dihydroanthracene, fluorene and 1,2,3,4-tetrahydronaphthalene) and a kinetic deuterium isotope effect (KIE) of 9.7 (k2(xanthene-h2)/k2(xanthene-d2)). On the basis of these kinetic data together with the DFT calculation results, we propose a stepwise reaction mechanism involving rate-limiting benzylic hydrogen atom abstraction and subsequent rebound of the generated organic radical intermediate to a remaining oxido group on the osmium centre.
O stretches as compared to those of tetrahedral OsO4 itself. Despite the negative shift of E1/2, the reactivity of these adduct complexes 1X was enhanced compared to that of OsO4 in benzylic C(sp3)–H bond oxidation. The reaction obeyed the first-order kinetics on both 1X and the substrates, giving the second-order rate constant (k2), which exhibits a linear correlation with the C–H bond dissociation energy (BDEC–H) of the substrates (xanthene, 9,10-dihydroanthracene, fluorene and 1,2,3,4-tetrahydronaphthalene) and a kinetic deuterium isotope effect (KIE) of 9.7 (k2(xanthene-h2)/k2(xanthene-d2)). On the basis of these kinetic data together with the DFT calculation results, we propose a stepwise reaction mechanism involving rate-limiting benzylic hydrogen atom abstraction and subsequent rebound of the generated organic radical intermediate to a remaining oxido group on the osmium centre.
- This article is part of the themed collection: Dalton turns 50 – celebrating our board members past and present


 Please wait while we load your content...
Please wait while we load your content...