Fluorescent small molecules with alternating triarylamine-substituted selenophenothiophene and triarylborane: synthesis, photophysical properties and anion sensing studies†
Abstract
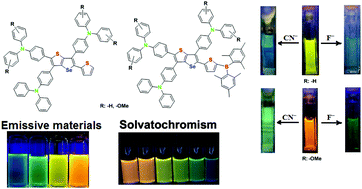
Two novel D–π-A fluorophores based on selenopheno[3,2-b]thiophene, possessing triphenylamine and 4,4′-dimethoxytriphenylamine units as donors and dimesitylborane as an acceptor, linked through a π-conjugated thiophene spacer (BTPAST and BOMeTPAST, respectively) were synthesized. Their photophysical properties were investigated in both solution and the state of aggregation and compared to those of their corresponding donor parts, having no dimesitylborane units (TPAST and OMeTPAST). All the compounds displayed large Stokes shifts between 100 and 140 nm with positive solvatochromism in solvents having different polarities. While BTPAST displayed both aggregation induced emission (AIE) and twisted intramolecular charge transfer (TICT) characteristics, the others preponderated with TICT effects. The sensing abilities of BTPAST and BOMeTPAST towards different anions were studied. Both exhibited chromogenic and fluorogenic responses to small anions such as fluoride and cyanide, for which the detection limits were found to be 0.12 and 2.43 ppm with BTPAST and 0.59 and 0.92 ppm with BOMeTPAST, respectively. These results provide guidance for the development of novel fused selenophenothiophene sensors in the field of anion sensing.


 Please wait while we load your content...
Please wait while we load your content...