Fluorescent organo-antimony compounds as precursors for syntheses of redox-active trimeric and dimeric alkali metal antimonides: an insight into electron transfer reduction processes†
Abstract
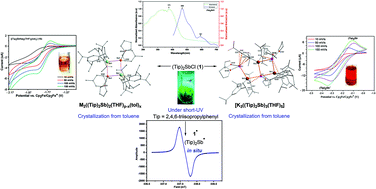
(Tip)2SbCl (1, Tip = 2,4,6-triisopropylphenyl) has been utilized as a precursor for the synthesis of the distibane (Tip)4Sb2 (4) via one-electron reduction using KC8. The two-electron reduction of 1 and 4 afforded the novel trinuclear antimonide cluster [K3((Tip)2Sb)3(THF)5] (6). Changing the reducing agent from KC8 to a different alkali metal resulted in the solid-state isolation of corresponding stable dimeric alkali metal antimonides with the general formula [M2((Tip)2Sb)2(THF)p−x(tol)x] (M = Li (14), Na (15), Cs (16)). In this report, different aspects of the various reducing agents [K metal, KC8, and [K2(Naph)2(THF)]] used have been studied, correlating the experimental observations with previous reports. Additional reactivity studies involving 1 and AgNTf2 (Tf = trifluoromethanesulfonyl) afforded the corresponding antimony cation (Tip)2Sb+NTf2− (19). The Lewis acidic character of 19 has been unambiguously proved via treatment with Lewis bases to produce the corresponding adducts 20 and 21. Interestingly, the precursors 1 and 4 have been observed to be highly luminescent, emitting green light under short-wavelength UV radiation. All the reported compounds have been characterized via NMR, UV-vis, mass spectrometry, and single-crystal X-ray diffraction analysis. Cyclic voltammetry (CV) studies of 1 in THF showed possible two electron reduction, suggesting the in situ generation of the corresponding radical-anion intermediate 1˙− and its subsequent conversion to the monomeric intermediate (Tip)2Sb− (5) upon further reduction. 5 undergoes oligomerization in the solid state to produce 6. The existence of 1˙− was proved using electron paramagnetic resonance (EPR) spectroscopy in solution. CV studies of 6 suggested its potential application as a reducing agent, which was further proved via the conversion of Tip–PCl2 to trimeric (Tip)3P3 (17), and cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P–Cl (cAAC = cyclic alkyl(amino)carbene) to (cAAC)2P2 (18) and 4, utilizing 6 as a stoichiometric reducing agent.
P–Cl (cAAC = cyclic alkyl(amino)carbene) to (cAAC)2P2 (18) and 4, utilizing 6 as a stoichiometric reducing agent.


 Please wait while we load your content...
Please wait while we load your content...