Selective olefin production on silica based iron catalysts in Fischer–Tropsch synthesis†
Abstract
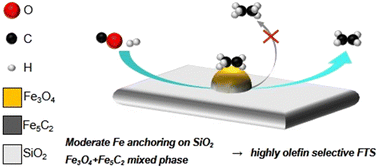
We demonstrated two design methods for obtaining Fe catalysts to improve the olefin selectivity in Fischer–Tropsch synthesis (FTS) reactions through the use of a SiO2 support. Fe/SiO2 catalysts, synthesized through classical impregnation, and Fe–SiO2 catalysts, synthesized through impregnation followed by calcination at 1700 °C, with Fe loadings of 1, 5, and 10 wt% were obtained. The Fe–SiO2 catalysts achieved C2–C4 olefins in C2–C4 hydrocarbons, O/(O + P), of 62.5–73.0%, whereas the O/(O + P) of the Fe/SiO2 catalysts decreased significantly with high Fe loadings. Using various characterization techniques, the main carbide species was identified to be Fe5C2, and based on the crystal size and composition of the Fe species, it was concluded that the interactions of Fe–SiO2 catalysts with SiO2 are of moderate strength. The iron time yield of C2–C4 olefins (FTYC2–C4 olefins) as a function of the Fe5C2 composition was higher for the Fe–SiO2 catalysts than for the Fe/SiO2 catalysts with corresponding Fe loadings; the highest value obtained for 1 wt% Fe–SiO2 was a factor of 3.5 higher than that of 1 wt% Fe/SiO2. The reaction results showed that the C2–C4 olefins can be selectively produced using the Fe–SiO2 catalyst even with a high Fe content. A density functional theory (DFT) study was performed to investigate the effect of SiO2 promotion on Fe carbides, and it was concluded that SiO2 promotion resulted in high selectivity for olefins, CH4, and CO2. To suppress CH4 or CO2 formation and boost CO conversion, Na-added catalysts with various Na ratios were tested, and successfully reduced CH4 selectivity and enhanced CO conversion.
- This article is part of the themed collection: Emerging Investigator Series


 Please wait while we load your content...
Please wait while we load your content...